Reaction Type Crystallizer
Reaction-Type Crystallizers In chemical reactions in which the end product is a solid-phase material such as a crystal or an amorphous solid, the type of equipment in Fig. hereinafter may be used. By mixing the reactants in a large circulated stream of mother liquor containing suspended solids of the equilibrium phase, it is possible to minimize the driving force created during their reaction and remove the heat of reaction through the vaporization of a solvent, normally water. Depending on the final particle size required, it is possible to incorporate a fines-destruction baffle as shown in Fig. and take advantage of the control over particle size afforded by this technique. In the case of ammonium sulfate crystallization from ammonia gas and concentrated sulfuric acid, it is necessary to vaporize water to remove the heat of reaction, and this water so removed can be re injected after condensation into the fines-destruction stream to afford a very large amount of dissolving capability.
Other examples of this technique are where a solid material is to be decomposed by mixing it with a mother liquor of a different composition, Carnallite ore (KClMgCl2'4H2O) can be added to a mother liquor into which water is also added so that decomposition of the ore into potassium chloride (KCl) crystals and magnesium chloride-rich mother liquor takes place.
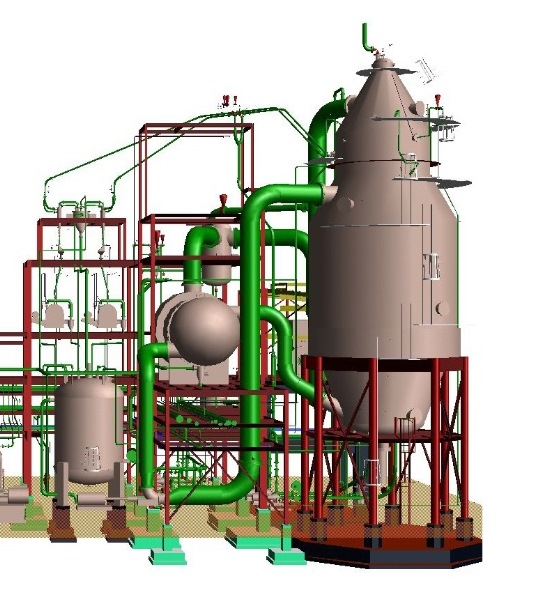
Reaction Type (DTB) Crystallizer
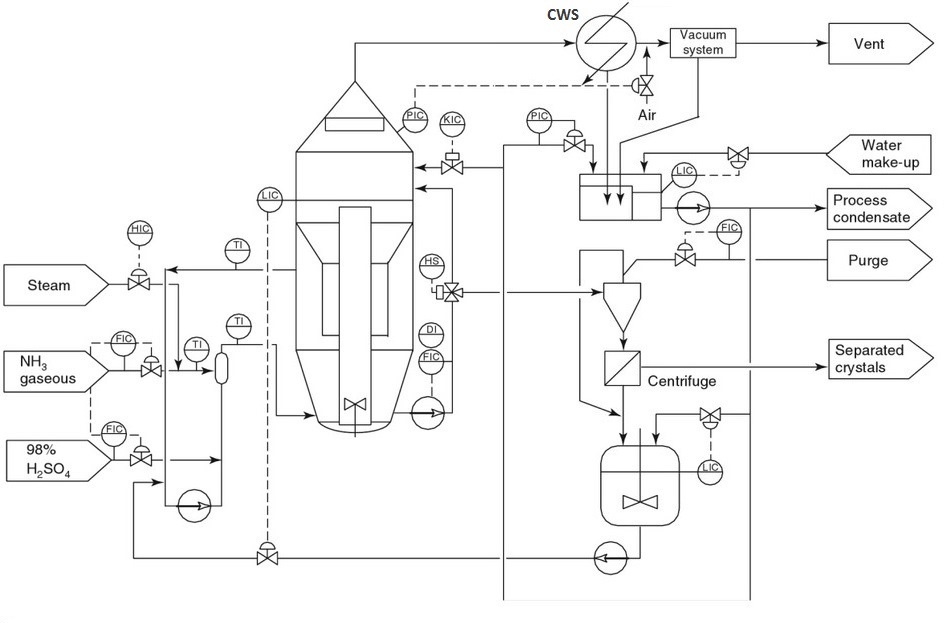
Reaction Type (DTB) Crystallizer for Ammonium Sulfate production

